Services
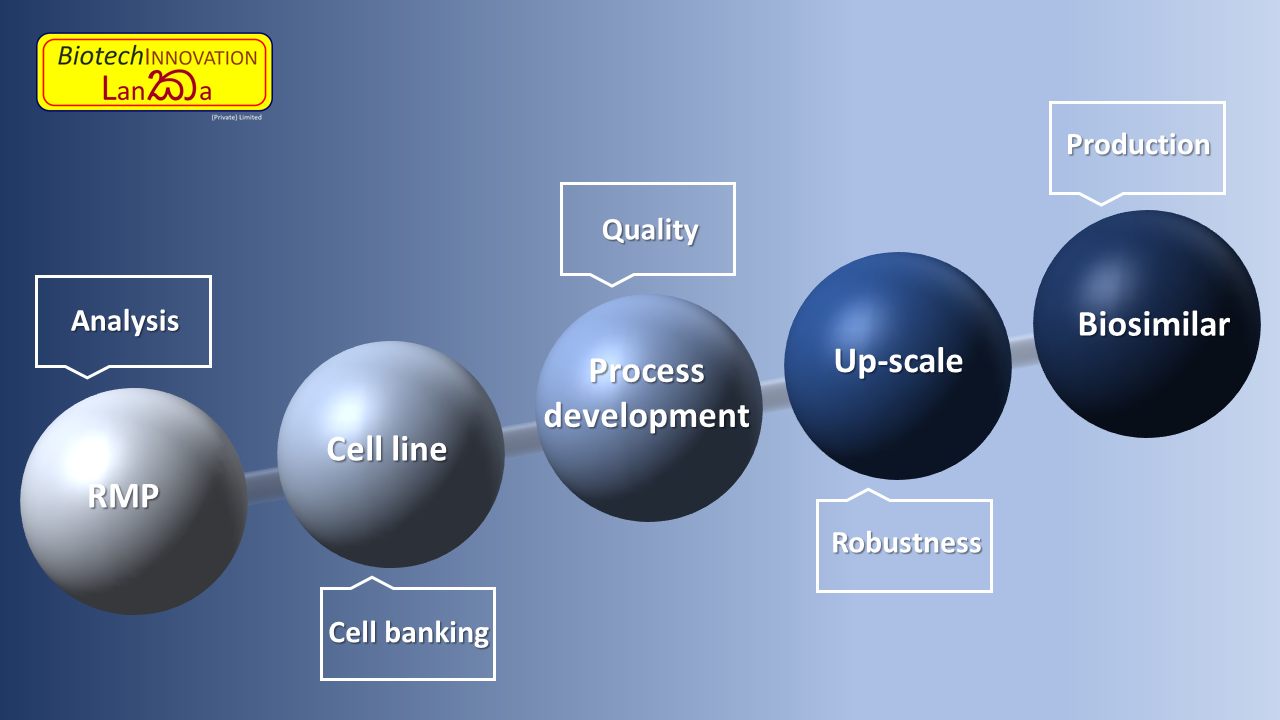
Our expertise's in Biotech-GMP consultancy:
- Biosimilar development (from lab scale to market)
- Support for USP, DSP, Analytics. Process development (GMP in mind)
- Process optimization, robustness, quality
- Technology transfers from Europe to Asia and vice versa
- Planing/setting-up of Production Facilities for IV solutions
- GMP conform documentation (design/writing/review of SOP's, validation plans/reports etc.)
- Support for GMP audits (from authorities or execution of external audits)
Consultant activities for biosimilars:
- Analysis of Reference Medical Product (RMP)
- Cell banking (generation/testing/release of MCB, WCB, EPC and PPC)
- USP, DSP development, up-scale, engineering batches, HCP generation
- Development of specific analytics: HCP ELISA, hDNA assay etc.
- Virus clearance study
- Establishment and characterization of reference/working standards
- Stability studies (DS, DP, RMP)
- Formulation development
- API production (DP toxicology study material, process validation, DP manufacturing)

Further consultancy services:
- Routine production: data interpretation, trends, trouble shooting
- Optimization of process robustness and efficiency
- Up-scale opportunities
- URS, IQ, OQ, PQ for downstream equipment
- Validation issues
- API storage, shipment
- SOP review
